Affiliations
Rationale
Subcutaneous immunotherapy has not only to be effective, but also needs to be safe and well tolerated in order to be accepted by physicians and patients. Here we show the safety data from 2 double-blind, placebo-controlled studies and 1 open follow-up study with mannan-conjugated birch pollen allergoids (T502).
Methods
The data of all patients who received pre-seasonal treatment with 10.000 mTU/mL T502 were analyzed. Safety and clinical tolerability were assessed by means of solicited and unsolicited adverse events (AEs) in all 3 studies and the data was then pooled.
Results

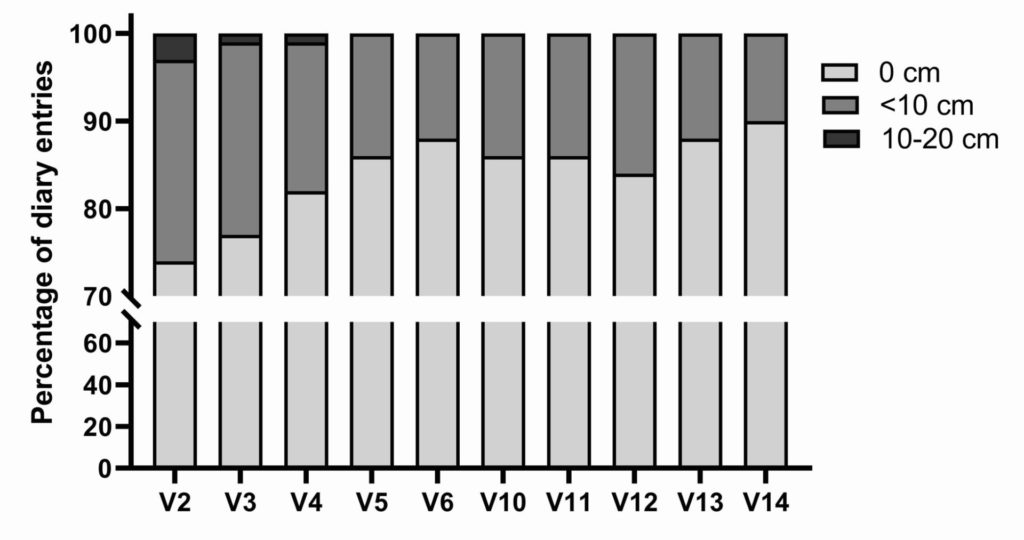
Data is expressed as percentage of diary entries per visit.
| AWMF-Grading | Placebo | T502 | ||
|---|---|---|---|---|
| N = 160 Pts | N = 1219 Inj. | N = 414 Pts | N = 3371 Inj. | |
| Grade I | 0.63% | 0.08% | 5.56% | 0.45% |
| Grade II | 0% | 0% | 2.97% | 0.24% |
| Grade III | 0% | 0% | 0% | 0% |
| Grade IV | 0% | 0% | 0% | 0% |
| TOTAL | 0.63% | 0.08% | 8.52% | 0.69% |
In total, 23 systemic reactions according to the German AWMF grading were reported by 19 patients (4.5%, N=419). Broken down to the number of T502 injections (N=3371), SRs occurred in 0.7% of injections.
| MedDRA Preferred Term | Placebo | T502 | ||
|---|---|---|---|---|
| N = 160 Pts | N = 1219 Inj. | N = 414 Pts | N = 3371 Inj. | |
| Injection site pruritus | 0.0% | 0.00% | 37.4% | 4.60% |
| Pruritus | 1.3% | 0.16% | 14.5% | 1.78% |
| Injection site reaction | 0.0% | 0.00% | 14.3% | 1.75% |
| Injection site swelling | 0.0% | 0.00% | 13.0% | 1.60% |
| Peripheral swelling | 0.0% | 0.00% | 12.3% | 1.51% |
| Injection site pain | 0.0% | 0.00% | 5.3% | 0.65% |
| Local reaction | 0.0% | 0.00% | 3.1% | 0.39% |
Data is expressed as percentage of MedDRA Preferred Term (according to the investigators documentation) per all patients of the respective treatment group and per all injections of the respective treatment.
Conclusions
Subcutaneous immunotherapy with T502 is safe and well tolerated with local and systemic reactions being comparable to other studies. A further dose split considerably improves the tolerability.
This work was funded by Inmunotek S.L.